Describe a Polar Covalent Bond Using the Term Electronegativity
The term covalent bond refers to a link formed by the sharing of electron pairs among distinct or similar kinds of molecules. We take this kind of Electronegativity Difference Bond graphic could possibly be the most trending topic once we portion it in google benefit or facebook.
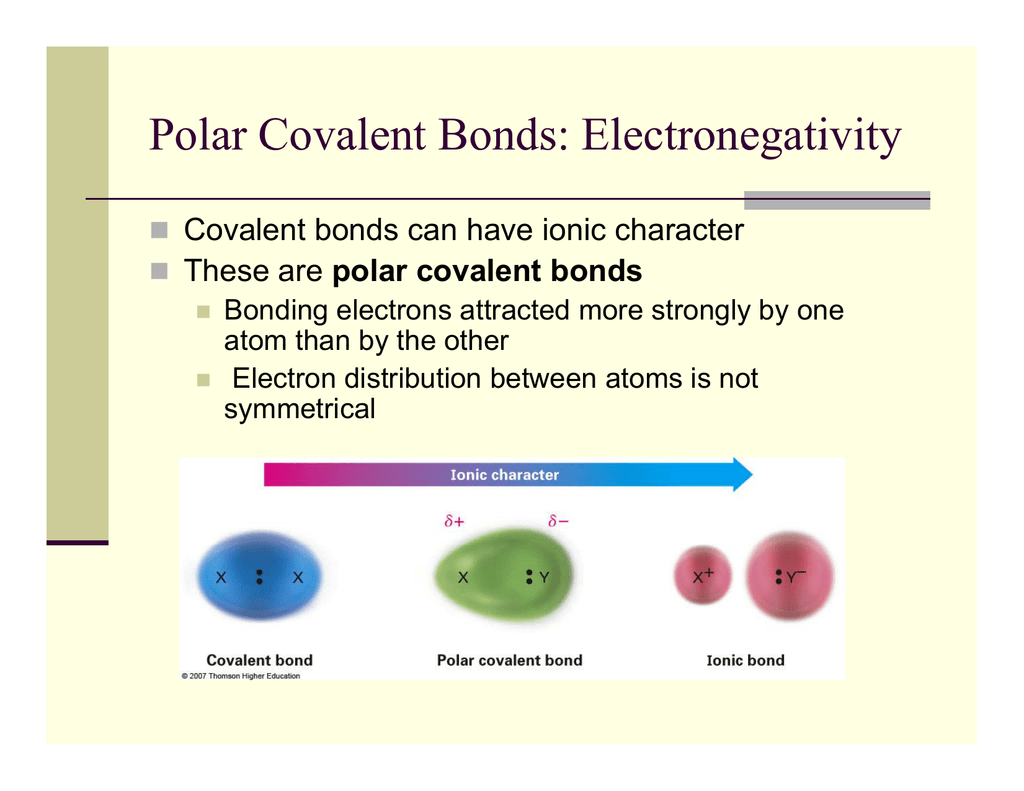
Polar Covalent Bonds Electronegativity
Pure covalent-electrons are being shared equally between atoms.
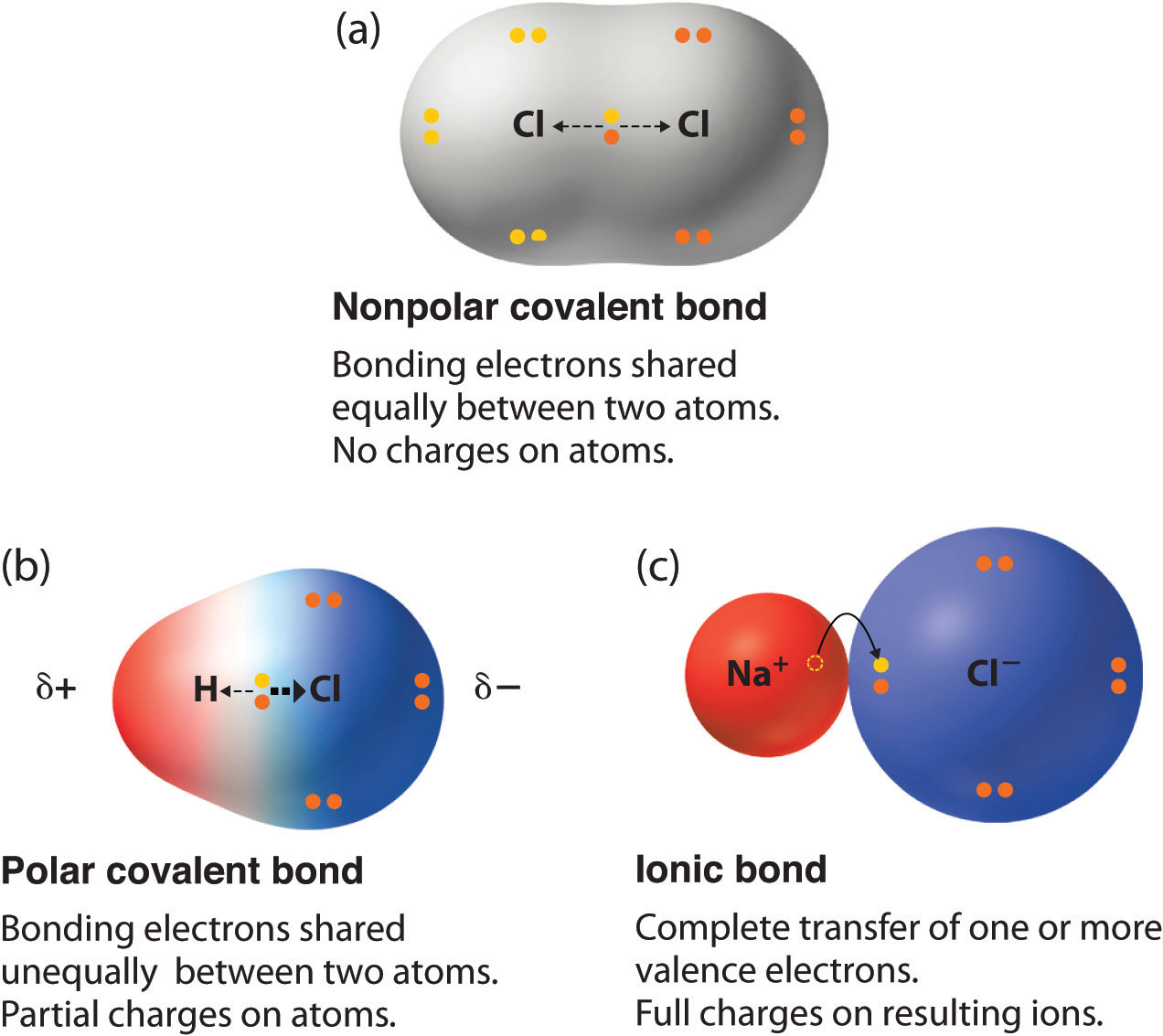
. I When X A X B ie. X A X B 0 then A-B bond is non-polar covalent bond or simply covalent bond and is represented as A-B. Its submitted by giving out in the best field.
For more information on Electronegativity Bond Scale Examples of polar covalent bonds The hydrogen fluoride HF molecule has a polar bond between the atoms. Answer 1 Covalent Bond is formed by sharing of electrons between two atoms Covalent bond is of two types- Polar Covalent Bond- When there is difference in electronegativity between the two non-metals. If the difference in electronegativity is greater than 17 the character of the bond will be ionic.
In the extreme we have an ionic bond. That is to say identical pairs of atoms form a nonpolar covalent bond. Polar covalent pure covalent electronegati.
Here are a number of highest rated Electronegativity Difference Bond pictures upon internet. The hydrogen-chlorine bond in HCl or the hydrogen-oxygen bonds in water are typical. We identified it from honorable source.
Describe polar covalent bonds of water based on the partial charges of the atoms Describe electronegativity using the definition of Linus Pauling Use electronegativity to distinguish between a polar covalent bond and a non-polar covalent bond Use electronegativity to distinguish between covalent and ionic bonds Expert Answer. The bond is nonpolar If the electronegativity difference is less than. A covalent bond with an equal share of electrons and an electronegativity difference of zero is called a nonpolar covalent bond.
Atoms with electronegativity differences between mathbf 04 and mathbf 17 form polar covalent. In other words the electrons spend more time on one side of the bond than the other. If the electronegativity difference is greater than 17 the bond is ionic.
A covalent bond with an unequal sharing of electrons and the electronegativity difference within the range of 01-2 is called a polar covalent bond. Of the two combining atoms. Polar covalent bonds are usually formed between two nonmetal atoms having different electronegativities.
In a polar covalent bond one atom spends more time with. The concept of electronegativity can be used to predict whether the bond between similar or dissimilar atoms is the non-polar covalent bond polar covalent bond or ionic bond. They form when the electronegativity difference between the anion and cation is between 04 and 17.
H-H bond in H 2 molecule is a. The more electronegative element will attract electron density towards itself resulting in uneven charge distribution. Examples include most covalent bonds.
If the ΔEN is greater than 20 then the bond is. Difference in EN of atoms 2 Ionic Bonds. A polar bond is a covalent bond in which there is a separation of charge between one end and the other - in other words in which one end is slightly positive and the other slightly negative.
If the electronegativity difference usually called ΔEN is less than 05 then the bond is nonpolar covalent. Polar bonds are intermediate between pure covalent bonds and ionic bonds. Bond Polarity and Inductive EffectBond Polarity and Inductive Effect Nonpolar Covalent Bonds.
Polar covalent-electrons are being shared unequally between atoms. Ionic-electrons have been transferred from one atom to another. Covalent Bond Examples There is a covalent bond between the oxygen and each hydrogen in a water molecule H 2 O.
If the electronegativity difference is between 04 and 17 the bond is polar. Difference in EN 2 CH bonds relatively nonpolar C-O C-X bonds more electronegative elements are polarelectronegative elements are polar Bonding electrons shift toward. What elements make polar and non-polar bonds.
If the ΔEN is between 05 and 16 the bond is considered polar covalent 3. This property is roughly described as electronegativity If two atoms of differing electronegativity form a bond the electrons spend more time on the more electronegative atom. A polar covalent bond is when two atoms are not sharing an electron equally.
In a nonpolar covalent bond the atoms share the electron equally. A polar bond is a type of covalent bond in which the electrons forming the bond are unequally distributed. Atoms with similar EN Polar Covalent Bonds.
The degree to which an atom attracts electrons in a chemical bond is described by electronegativity. Electronegativity Bond Scale Using the Pauling electronegativity bond scale a polar covalent bond has an electronegativity difference between 04 to 17. Let us consider A and B in which them is electronegativity difference is not equal to zero contains a covalent bond between them.
To remember a polar covalent bond instead say puller covalent and remember one atom has more pull on electrons than the other atom. Terms in this set 64 Match each type of bond to the disposition of valence electrons between atoms. Is called a polar covalent bond.
So lets review the rules. A polar bond refers to a type of covalent bond in which electrons are not equally shared and electronegativity values are slightly different. The shared pair of electrons forming a bond between A and B move towards move electronegative B.
Use electronegativity to classify bonds as polar covalent pure covalent or ionicTopics. A polar covalent bond is formed when the electronegativity difference is between 5 and 17. A polar bond is a bond between two atoms of varying electronegativity.
Electronegativity Polar Covalent Bonds. If the difference in electronegativity is between 04 and 17 the character of the bond is polar covalent. Some elements tend to attract electrons more strongly than others.

2 1 Polar Covalent Bonds Electronegativity Flashcards Quizlet
Ppt 2 1 Polar Covalent Bonds Electronegativity Powerpoint Presentation Id 2237583
No comments for "Describe a Polar Covalent Bond Using the Term Electronegativity"
Post a Comment